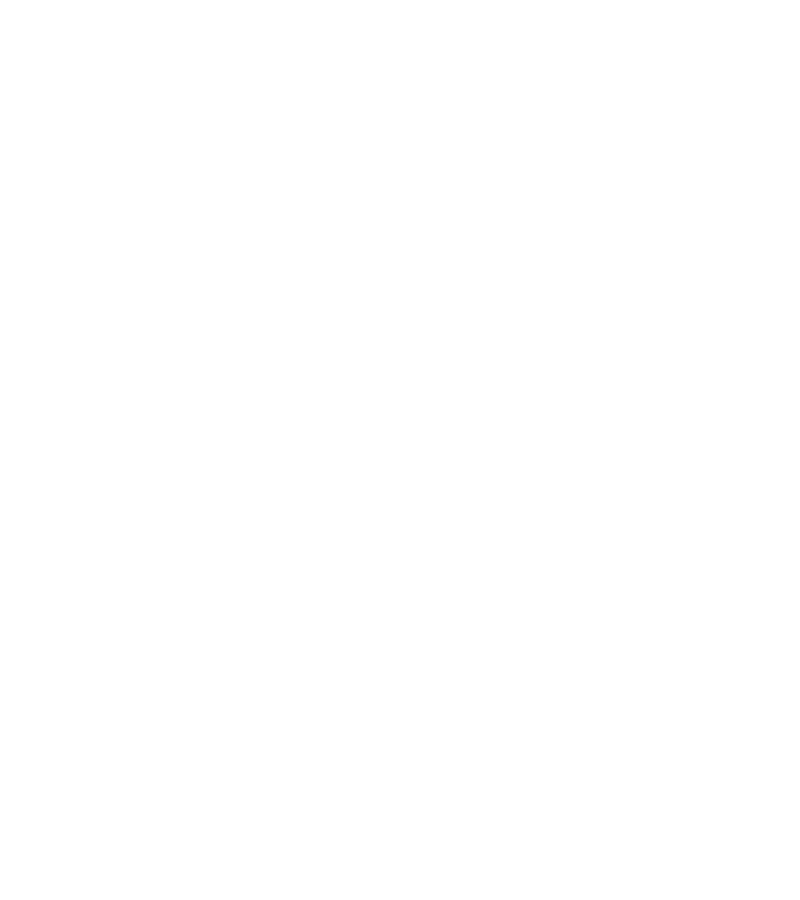
Bypassing The Blood-Brain Barrier in CNS Therapeutic Delivery
Intranasal delivery to the olfactory epithelium bypasses the blood-brain barrier (BBB), a failure of many neurologic/CNS therapies, thereby improving drug delivery.
People sustain a TBI in the US every year
Annual costs to US society for care and lost productivity
Time to recover for 80-90% of concussed patients
Complain of sleep disturbances
Rate of female athletes suffering concussions vs male athletes
Suffer from a TBI
People are diagnosed with ALS in the US every year
Average life expectancy for patient diagnosed with ALS
Annual costs to US society for care and lost productivity

ALS is always fatal
Increased incidence of +70% by 2040
Have a 2X higher risk of developing ALS

Co-Founder & CEO, brings over thirty years experience in the pharma industry. At GSK and Teva, he led respiratory marketing for multiple brands including ProAir, Flonase, Serevent, QVAR, QNASL, and other products with annual portfolio sales over $1 billion (Teva). His leadership roles have encompassed marketing, brand management, product launch, pricing, and payer strategy. Earlier in his career, he was a brand manager for Procter & Gamble and legislative staff manager for Senator Bob Dole. BS, Kansas State University, MBA, Harvard Business School.

Co-Founder & Executive Chairman, has founded and led innovative respiratory technology companies in disease management, diagnostic instruments, and pharmaceuticals. Most recently, he founded and led a startup that has developed an intranasal epinephrine product for treatment of anaphylaxis, which is currently in human trials. He holds multiple patents in the respiratory field encompassing diagnostic, therapeutic and informatic applications. BA Yale University, MBA. University of Virginia.

Co-Founder & Chief Medical Officer, has been treating patients with ALS and other neurological conditions for over twenty-five years. His practice has included the treatment of traumatic brain injury, autism, pediatric orphan diseases. Dr. Lombard’s extensive clinical experience and deep understanding of pathological and biochemical processes underlying neurological disease are foundational in Precedent’s product development. He served as Chief of Neurology at Bronx Lebanon Hospital, and his academic appointments have included instruction in neurology at New York Presbyterian Hospital and Albert Einstein College of Medicine. DO Nova Southeastern University, BS Florida Atlantic University.

Leigh Berryman is an executive and scientist in the CRO and biopharma industries across North America, Europe, and Asia. As an accredited toxicologist, he has contributed to over 350 new drug clinical trial applications (preclinical) and first in man programs. He was founder of LAB Research and CEO of Maccine Pte. Ltd., and has raised over $200M innew funding and played a pivotal role in six mid-sized M&A transactions. Since 2014, he has worked with early-stage companies to design development programs with a focus on cost and timing to enable evaluation of risk and return on required investment capital. B.S, Univ. of Newcastleon Tyne, MRB. CBiol. RQAP-GLP, Reg. Toxicologist.

Dr. Hanson is a former USAF flight surgeon with extensive training in life support and operational medicine, epidemiology, and expertise in feasibility assessment of novel medical technologies. His expertise encompasses treatment for TBI, brain imaging, and battlefield injury assessment and care management. His operational experience includes over 750 hours in a range of aircraft and deployments in Desert Storm/Desert Shield, Bosnia and other actions. He served as Division Chief, Science & Technology, US Air Force Medical service, where he managed the review/award of several hundred medical research grants under the $1.4B CMRDP program, Senior Flight Surgeon, and Chief, Operational Biotechnology and Genomics in the US Surgeon General’s Office. MD, Uniformed Services Medical University, MPH, Johns Hopkins University.

Mr. Monaco is a business and medical Informatics expert with extensive experience in project management, systems engineering, clinical trial design, and other life sciences disciplines. His clients have included Biogen, J&J, AstraZeneca, CVS, United Health, Ascension Healthcare, Allegheny Health. Most recently, he directed technical validation and verification projects for exome and whole genome sequencing for the Broad Institute of Harvard and MIT. This is led to the successful integration and commercialization of sequencing technologies from Illumina and Pac Bio, and AI/supercomputing capabilities from Google and AWS. BS and MS in Civil Engineering, Carnegie Mellon University

Professor & Chair, Dept. of Pharmacy Practice, University of New Mexico. Professor Emeritus, U. Michigan College of Pharmacy. He has conducted a wide range of pharmacologic and clinical research, including investigations related to cardiovascular disease and heart failure. Dr. Bleske performed the first research studies of the intranasal delivery of epinephrine. PharmD University of Minnesota, BS Wayne State University.

Mr. Oates is President of the NFL Alumni Association and leading advocate for NFL alumni health. He is a three-time Super Bowl champion, and Pro Bowl Center for the New York Giants and San Francisco 49ers professional football teams. BS BYU, JD Seton Hall.

Ms. Dunn is co-founder of BlueGene Search, an executive search agency in the biotech and rare disease space. She is a subject matter expert on relationships with patient advocacy organizations working in conditions with few, if any, therapeutic options. BA, Towson University.

Professor Holman teaches intellectual property law with a focus on biotechnology at the University of Missouri – Kansas City. He has published numerous articles in law reviews and scientific publications such as Science, Cell and Nature Biotechnology, and has authored amicus briefs in a number of important biotechnology patent cases at the Supreme Court and Federal Circuit. Prior to joining the UMKC faculty in 2005, he served as patent counsel at several Silicon Valley biotech firms and a major IP law firm. After completing his Ph.D. in biochemistry and molecular biology, he engaged in post-doctoral drug discovery research at Roche Biosciences. PhD, U. California, Davis, JD, U. California, Berkeley.


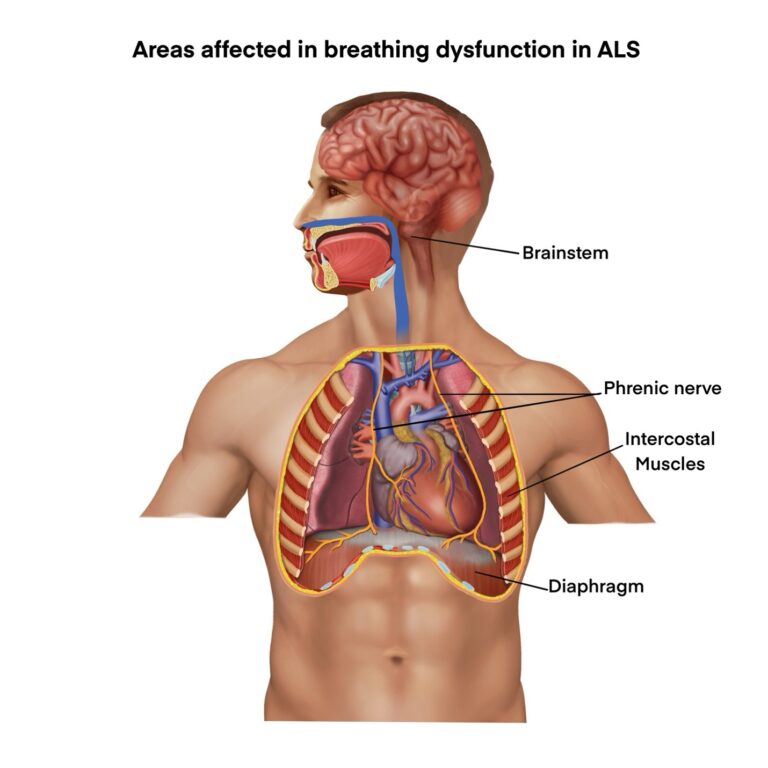
Precedent’s PTx-002 Candidate addresses respiratory dysfunction in ALS – the primary cause of mortality in ALS

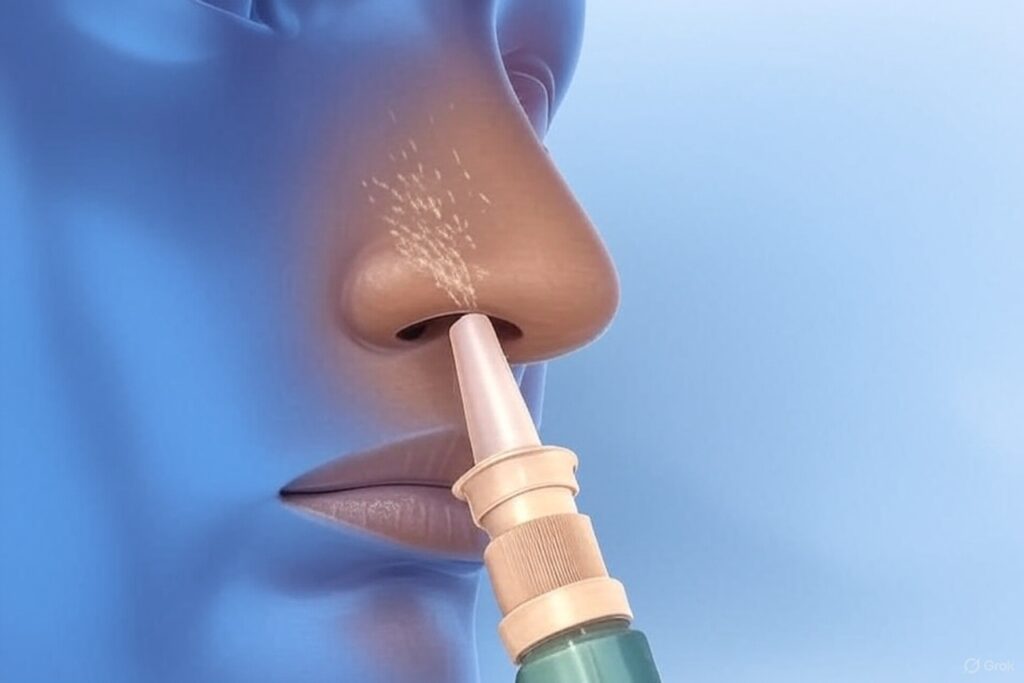
Precedent formulates drug candidates for intranasal delivery to better target drug delivery,
improve bioavailability and increase ease of use for patients and caregivers